Letters | Regulation of Hong Kong’s beauty industry cannot be skin deep
Readers discuss the need for stricter oversight of a fast-growing sector, the proper usage of the archaic ‘cum’, and Trump’s plan to cut the budget deficit
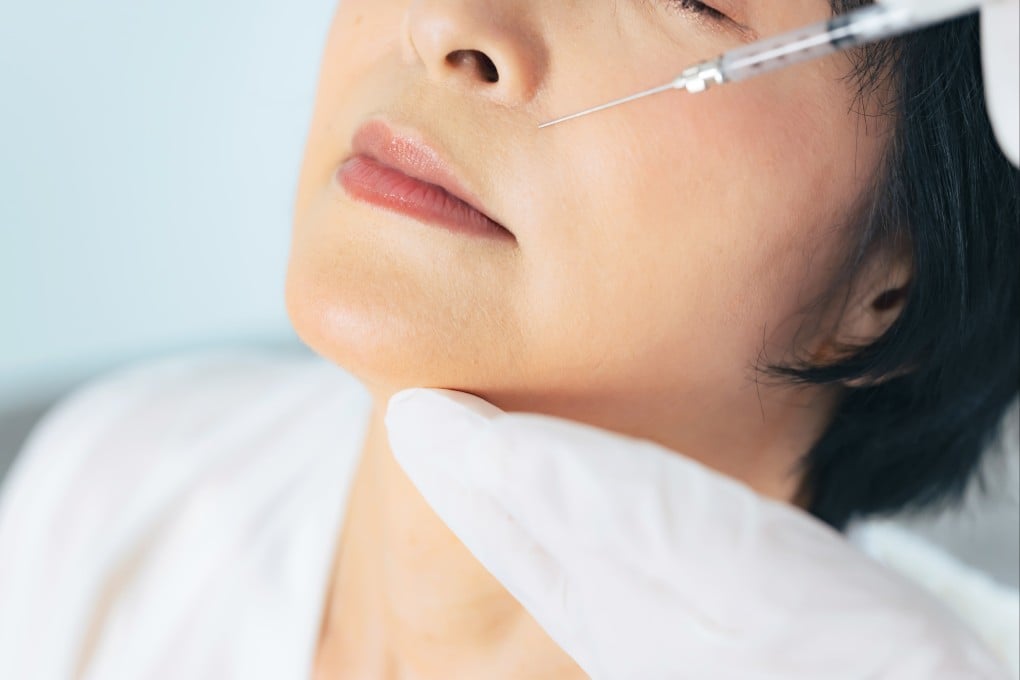
Hong Kong’s beauty and personal care sector is estimated to have grown into a US$2.38 billion market this year, and is expected to expand steadily at 2.35 per cent annually until 2030. Meanwhile, the beauty services industry – comprising hairdressing, skincare, manicure, slimming services and the like – generated revenue of HK$7.3 billion as far back as 2015, according to one market study.
In November 2019, a Legislative Council joint subcommittee published a paper focusing mainly on government measures, such as funding and development initiatives, to support the industry. These measures were taken before any clear regulatory framework or quality assurance mechanisms – except perhaps in the area of training – were put in place.
This raises the question: why prioritise the proliferation of small and medium-sized enterprises in the beauty sector before ensuring the safety, efficacy and standards of the products and services offered? Consumers are spending their hard-earned money on treatments that may not deliver promised results – and in some cases, may even pose health risks.
The subcommittee issued a report on its work in December 2019 with recommendations that on the regulatory front included distinguishing between medical and beauty devices, and communicating with stakeholders on the listing mechanism for medical devices. There doesn’t seem to have been much progress after that.